New Mechanistic Links Between SARS-CoV-2 and Alzheimer’s Disease
Triple Threats to α7 nAChRs
🧠 Introduction
Emerging evidence implicates SARS-CoV-2 as a potent disruptor of the cholinergic system—specifically, the alpha-7 nicotinic acetylcholine receptor (α7 nAChR). This receptor is critical for neuroimmune regulation and memory function and has been extensively studied in Alzheimer’s disease (AD) pathophysiology.
While Carlo Brogna’s pioneering work on gut microbiome-derived toxin-like peptides has already raised alarms, new experimental and computational studies show that the spike protein itself—not merely bacterial byproducts—can directly bind and dysregulate α7 nAChR. Together, these findings suggest a double and potentially triple-hit mechanism linking SARS-CoV-2 exposure to accelerated neurodegeneration.
🔬 The Basal Forebrain Cholinergic System in Alzheimer’s Disease
The basal forebrain cholinergic system is among the first neuronal networks compromised in AD, resulting in:
Declining levels of acetylcholine (ACh)
Loss of cholinergic neurons
Reduced expression and function of α7 nAChRs
Under physiological conditions, α7 nAChRs bind amyloid-beta (Aβ) with high affinity, facilitating neurotransmission and synaptic plasticity. However, in AD, pathologically elevated Aβ leads to receptor-mediated toxicity, pro-inflammatory signaling, and synaptic dysfunction.
Importantly, α7 nAChRs are also highly expressed in astrocytes and microglia, where they regulate neuroinflammation via the cholinergic anti-inflammatory pathway. Loss or inhibition of this receptor compromises innate immune homeostasis—exacerbating neurodegeneration.
🦠 Toxin-Like Peptides from the Gut Microbiome: Brogna’s Findings
Carlo Brogna and colleagues identified a novel class of toxin-like peptides generated by gut bacteria in response to SARS-CoV-2 infection -
Structure & Origin: Small cysteine-rich peptides, structurally similar to conotoxins and bungarotoxins, were detected in plasma, urine, and feces of COVID-19 patients. They are produced by commensal bacteria (e.g., Ruminococcus) in response to viral RNA or spike protein.
Receptor Binding: In vitro assays demonstrated high-affinity binding to α7 nAChRs, suggesting that these peptides act as antagonists—disrupting acetylcholine signaling in neurons and immune cells.
Correlation with Severity: Peptide abundance correlated with disease severity and persistence (Long COVID), supporting their role as mediators of cholinergic dysfunction and chronic inflammation.
Therapeutic Implications: Early data suggest microbiome modulation—via specific antibiotics or probiotics—may reduce peptide production.
https://doi.org/10.3390/biomedicines11010087
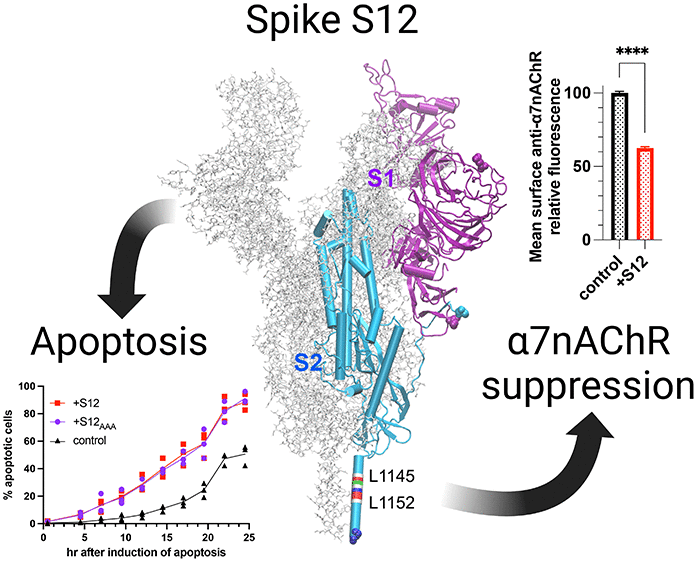
🧬 Direct Interaction of SARS-CoV-2 Spike Protein with α7 nAChRs
Recent mechanistic studies now indicate that the spike protein—particularly the S1 subunit and receptor-binding domain (RBD)—binds α7 nAChRs directly.
Key Findings:
Modulation of Receptor Function
Spike-derived peptides can both potentiate and inhibit receptor currents depending on concentration.
Agonist/Antagonist Duality
The Y674-R685 region exerts dual effects on receptor activity.
Inhibition of Surface Expression
A spike protein helix (S12, L1145–L1152) may prevent receptor trafficking.
In Silico Evidence
Molecular simulations show stable binding of spike peptides to α7 nAChR.
⚠️ A Triple Whammy: Converging Mechanisms of Dysfunction
Three overlapping insults appear to converge on α7 nAChR:
Endogenous Amyloid-Beta Overproduction – In predisposed individuals
Microbiome-Derived Toxins – From bacterial response to SARS-CoV-2
Direct Spike Binding – Impairing signaling and receptor expression
This cascade may explain accelerated cognitive decline or neuroinflammation in post-COVID patients—particularly those already vulnerable to neurodegenerative conditions.
🧪 Autoantibodies Against α7 nAChR: A Fourth Mechanism?
A 2024 study published in Translational Psychiatry adds a compelling dimension to α7-nAChR vulnerability. Researchers measured serum autoantibodies (AAbs) against α7-nicotinic receptors in patients with bipolar disorder and schizophrenia, uncovering a “continuum” of antibody levels tied to inflammation.
Antibody Mechanism: These α7 AAbs bind the receptor and enhance TNF-α release from macrophages—mimicking inflammatory signaling associated with viral persistence.
Clinical Relevance: Elevated AAb levels were associated with specific subgroups, linking α7-nAChR autoimmunity to neuroimmune dysregulation.
Implication: These findings reinforce concerns that persistent immune activation (e.g., post-viral) could drive similar autoantibody profiles in Long COVID.
📄 Read the full study (Darrau et al., 2024)
Link to article – Nature Translational Psychiatry
Figure 3: Hierarchical clustering of 5 inflammatory subtypes.
Group A (left) shows a strong pro-inflammatory profile (e.g., elevated IL-1β, IL-17, TNF-α), enriched in psychiatric cases. Group B (right) shows low/no inflammation and contains a higher proportion of healthy controls. Autoantibodies against α7-nAChR were detected in specific clusters with elevated cytokines.
🧠 Therapeutic and Research Directions
Key Priorities:
Animal model validation of spike/peptide binding to α7 nAChR
AAb screening in Long COVID cohorts
Trials of α7 nAChR-targeting therapeutics
Microbiome interventions (prebiotics, antibiotics, probiotics)
Potential Interventions:
α7 nAChR agonists (e.g., varenicline, nicotine patches)
Positive allosteric modulators
Microbiome rebalancing protocols
🧩 Conclusion
The evidence is mounting: α7 nAChR is under siege from multiple fronts in the aftermath of SARS-CoV-2 exposure. These findings call for a deeper understanding of neuroimmune interactions and the long-term implications of spike protein persistence.
When three mechanistic insults align—and a fourth looms—we can no longer ignore the possibility that SARS-CoV-2 is not only a respiratory virus, but a neurological disruptor.